37 redox reactions worksheet with answers
WS # 4 Balancing Redox Reactions . Balance each of the following half-cell reactions. (In each case assume that the reaction takes place in an ACIDIC solution.) Also, state whether the reaction is oxidation or reduction. 1.
View Act 3 Worksheet Types of REDOX Reaction (1).docx from CHEMISTRY 111 at Western Mindanao State University - Zamboanga City. NAME: _ Course & Year: _ Score: _/105_ Schedule: _ Date Performed: _
Sep 19, 2021 · Quiz & Worksheet - Election of 1824 & John Quincy Adams Quiz & Worksheet - Synopsis and Analysis of Robinson Crusoe Quiz & Worksheet - Redox Reactions & Electron Carriers in Cellular Respiration

Redox reactions worksheet with answers
nitrogen compounds, periodicity, polymerization, rates of reaction, reaction kinetics, redox reactions and electrolysis, states of matter, transition elements worksheets for college and university revision guide. "A Level Chemistry Quiz Questions and Answers" PDF download with
polymerization, rates of reaction, reaction kinetics, redox reactions and electrolysis, states of matter, transition elements worksheets for college and university revision guide. "A Level Chemistry Quiz Questions and Answers" PDF download with free
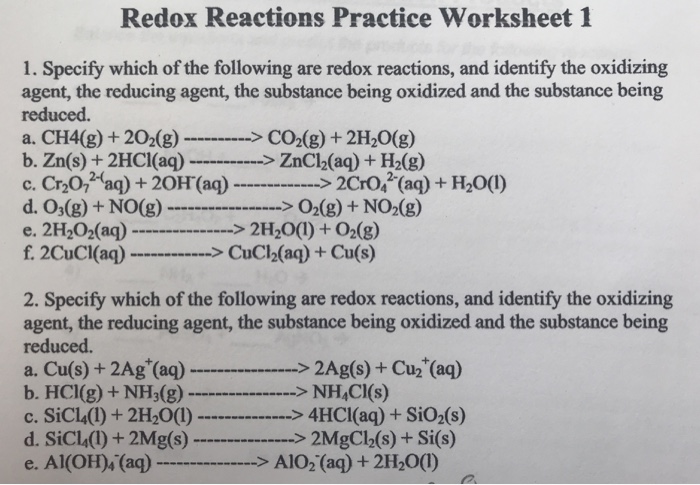
Practice Problems: Redox Reactions. Determine the oxidation number of the elements in each of the following compounds: a. H 2 CO 3 b. N 2 c. Zn(OH) 4 2-d. NO 2-e. LiH f. Fe 3 O 4 Hint; Identify the species being oxidized and reduced in each of the following reactions: a. Cr + + Sn 4+ Cr 3+ + Sn 2+ b. 3 Hg 2+ + 2 Fe (s) 3 Hg 2 + 2 Fe 3+ c. 2 As ...
Redox reactions worksheet with answers.
Redox reactions answer key determine the oxidation number of the elements in each of the following compounds. In the reaction 2k cl2 2kcl the species. Balance the reaction and indicate which reactant is oxidized and which reactant is being reduced. Identify the species being oxidized and reduced in each of the. Redox practice worksheet name.
Redox Reactions Worksheet Answer Key. Mg0 2H1 Cl-1 Mg1 Cl2-1 H20 Mg is oxidized RA. Determination of activity of some metals by reaction with hydrogen ion doc 28 kb redox. In the reaction mg cl2 mgcl2 the correct half reaction for the. H 2o 2 cr 2o 7 2 o 2 cr 3 9. 10h 4zn no 3 4zn2 nh 4 3h 2o 3. Balance each of the following half cell reactions.
Practice: Redox reactions questions. This is the currently selected item. Oxidizing and reducing agents. Disproportionation. Worked example: Balancing a redox equation in acidic solution. Worked example: Balancing a redox equation in basic solution.
This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and combustion reactions. For the first few reactions, the type of reaction is listed, you should predict the products, then balance. Further questions just have
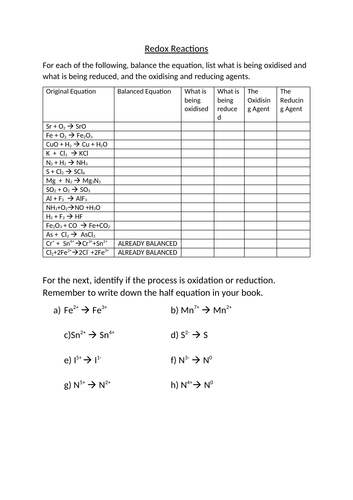
Chapter 20 Worksheet: Redox I. Determine what is oxidized and what is reduced in each reaction. Identify the oxidizing agent and the reducing agent, also. 1. 2Sr + O2 2SrO 2. 2Li + S Li2S 3. 2Cs + Br2 2CsBr 4. 3Mg + N2 Mg3N2 5. 4Fe + 3O2 2Fe2O3 6. Cl2 + 2NaBr 2NaCl + Br2 7. Si + 2F2 SiF4 8. 2Ca + O2 2CaO 9.
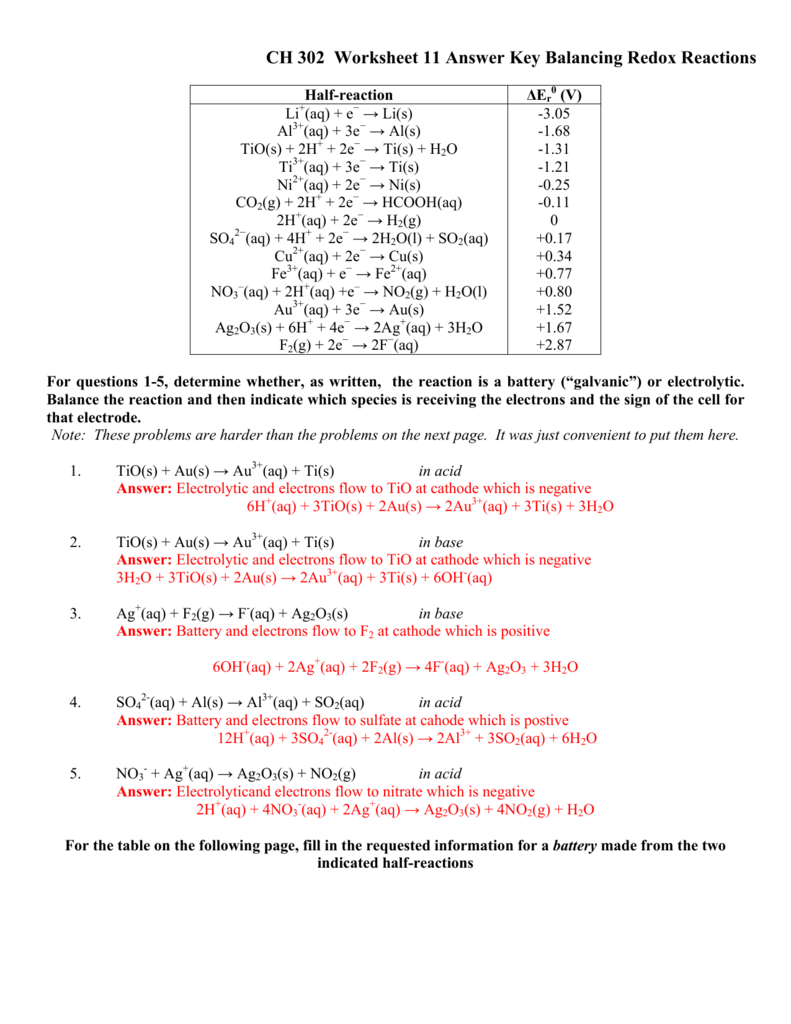
Balancing Redox Reactions via the Half-Reaction Method. Redox reactions that take place in aqueous media often involve water, hydronium ions, and hydroxide ions as reactants or products. Although these species are not oxidized or reduced, they do participate in chemical change in other ways (e.g., by providing the elements required to form ...
Write balanced equations for the following redox reactions: a. 2 NaBr + Cl 2 2 NaCl + Br 2 b. Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 in acidic solution c. 5 CO + I 2 O 5 5 CO 2 + I 2 in basic solution ; Write balanced equations for the following reactions: a. Cr(OH) 3 + Br 2 CrO 4 2-+ Br-in basic solution 10 OH-+ 2 Cr(OH) 3 + 3 Br 2 2 CrO 4 2-+ 8 H 2 O ...
Read PDF Redox Reaction Problems With Answer Key Grade 9 Biology Multiple Choice Questions and Answers (MCQs) (Book 1) - Introduction to Biology Quiz Questions and Answers (Book 2) - Biodiversity Quiz Questions and Answers (Book 3) - Bioenergetics Quiz Questions and Answers (Book 4) - Cell Cycle Quiz Questions and Answers (Book 5) - Cells and ...
Oct 21, 2021 · Redox Reactions. When we consider metabolism, which is the chemical processes of the body, oxidation and reduction reactions are best friends, meaning one cannot exist without the other.Because of ...
Balancing redox reactions worksheet 1 balance each redox reaction in. Balance the charge or oxidation number with electrons. In which substance is the oxidation number of nitrogen zero. Identify the pair of elements undergoing oxidation and reduction by checking oxidation states.
Recognizing redox reactions worksheet answers. 3mg n2 mg3n2 5. Balance each of the following half cell reactions. Suited for student in y10 and y11. In the reaction mg cl2 mgcl2 the correct half reaction for the oxidation that occurs is a. Redox reactions are a chemical reaction in which electrons are exchanged through oxidation and reduction.
Worksheet # 5 Balancing Redox Reactions in Acid and Basic Solution Balance each half reaction in basic solution. 4. Cr 2O 7 2 - → Cr3+ 5. NO → NO 3-6. SO 4 2- → SO 2 7. MnO 2 → Mn 2O 3 Balance each redox reaction in acid solution using the half reaction method. 8. H 2O 2 + Cr 2O 7 2- → O 2 + Cr 3+ 9. TeO 3 2-+ N 2O 4 → Te + NO 3-10 ...
Questions pertaining to redox reactions. 1 in combination with nonmetals o n. Cr 2o 7 2 cr3 5. No no 3 6. Balancing redox reactions worksheet 1. Clo3 cl æ cl2 clo2. 1 in all compounds 2. A change in phase. H 2o 2 cr 2o 7 2 o 2 cr 3 9. Choose a method and complete questions 12 13.
Predicting redox reactions using the half reaction table 1. Redox reactions worksheet. Cr 2o 7 2 cr3 5. Mn 2 bio3 æ mno4 bi 3 mno4 s2o3 2 æ s4o6 2 mn 2. In the reaction 2k cl2 2kcl the species. In the reaction al0 cr3 al3 cr0 the reducing agent is a. Ws 4 balancing redox reactions. 3mg n2 mg3n2 5. 2cs br2 2csbr 4. 5 2 customer reviews.
24. In the reaction Mg+Cl2!MgCl2, the correct half-reaction for the oxidation that occurs is A. Mg+2e !Mg2+ B. Cl2 +2e !2Cl C. Mg !Mg2+ +2e D. Cl2!2Cl +2e 25. The reaction that takes place in a chemical cell is best classi ed as A. fusion B. redox C. transmutation D. cracking 26. Which equation represents the half-reaction that takes place at ...
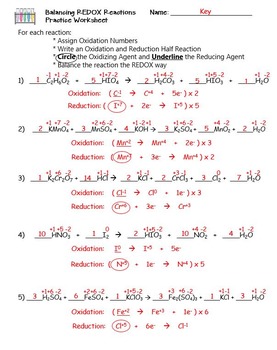
For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, and the reducing agent. 1) Mg + 2HCl ( MgCl2 + H2. 2) 2Fe + 3V2O3 ( Fe2O3 + 6VO. 3) 2KMnO4 + 5KNO2 + 3H2SO4 ( 2MnSO4 + 3H2O + 5KNO3 + K2SO4. ... Oxidation Reduction Worksheet Answers ...
Redox Reactions Answer Key Redox Reactions CHEM 10 Review Worksheet The questions on this worksheet are both Chem 10 and Chem 11 level questions. The reaction that takes place in a chemical cell is best classi ed as A. Redox Reactions Worksheet Pdf Printable worksheets are a valuable lecture room tool. Oxidizedreducing agent O0 to O2-.
Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
Worksheet 1 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
! 211!! ThehalfJreaction!method!involves!balancing!the!oxidation!reaction!as!if!it! wereanisolatedreaction.Thenthereductionhalf Jreaction!isbalancedasifit!were
Balancing Redox Reactions Worksheet 1 Balance each redox reaction in . acid. solution. Mn 2+ + BiO3 -Æ MnO4 -+ Bi 3+ MnO4 -+ S2O3 2- Æ S4O6 2- + Mn 2+
Oxidation Reduction Worksheet. Determine the oxidation number of each atom in the following substances. NF3 N +3 F -1 K2CO3 K +1 C 4 O -2 c. NO3- N____+5_____ O____-2_____ HIO4 H +1 I +7 O -2 For the following balanced redox reaction answer the following questions. 2 Fe+2(aq) + H2O2(aq) ( 2Fe+3(aq) + 2 OH-1(aq) What is the oxidation state of ...
Balancing REDOX Reactions: Learn and Practice Reduction-Oxidation reactions (or REDOX reactions) occur when the chemical species involved in the reactions gain and lose electrons. Oxidation and reduction occur simultaneously in order to conserve charge. We can "see" these changes if we assign oxidation numbers to the reactants and products.
admin November 18, 2019. Some of the worksheets below are Redox Reactions Worksheets, useful trick to help identify oxidation and reduction, step by step guide to balance any Redox Equations, explanation of Oxidation, reduction, oxidizing agent, reducing agent and rules for assigning an oxidation number, …. Once you find your worksheet (s ...
Aug 16, 2021 · Redox Reactions & Electron Carriers in Cellular Respiration: Definitions and Examples Quiz Instructions: Choose an answer and click 'Next'. You will receive your score and answers at the end.
Aug 26, 2021 · Quiz & Worksheet - Methods of Parenting Quiz & Worksheet - Reforms in the Late Roman Republic Quiz & Worksheet - Redox Reactions & Electron Carriers in Cellular Respiration
reaction and reduction half-reaction, identify the oxidizing and reducing agents and any spectator ions. Half - Reactions Homework ... Balance the following redox reactions by the half-reaction method, rewriting the balanced equations below the given unbalanced equation. Show your work below each reaction and
Balancing Redox Equations Method 2: Half-reaction method 1. Divide the skeleton reaction into two half-reactions, each of which contains the oxidized and reduced forms of one of the species 2. Balance the atoms and charges in each half-reaction - Atoms are balanced in order: atoms other than O and H, then O, then H
Balancing redox reactions in basic solution. If the redox reaction was carried out in basic solution (i.e. alkaline conditions), then we have to put in an extra step to balance the equation. The steps for balancing redox reactions in basic solution are: Identify the pair of elements undergoing oxidation and reduction by checking oxidation states




















![2-5 Redox Reactions Practice Worksheet With Answers [klzzqq3gwqlg]](https://idoc.pub/img/crop/300x300/9n0kgpw6zk4v.jpg)




0 Response to "37 redox reactions worksheet with answers"
Post a Comment