38 mole calculations practice worksheet answers
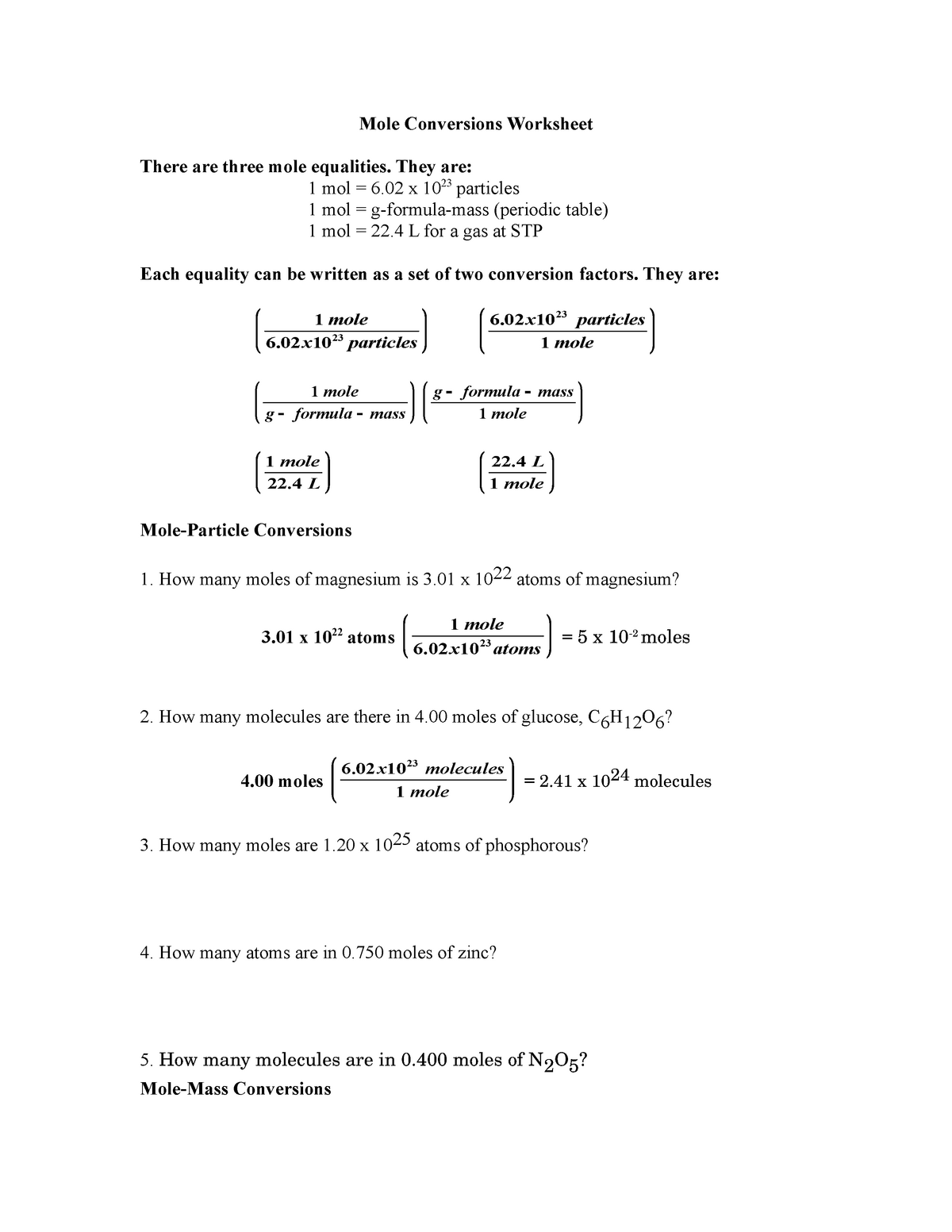
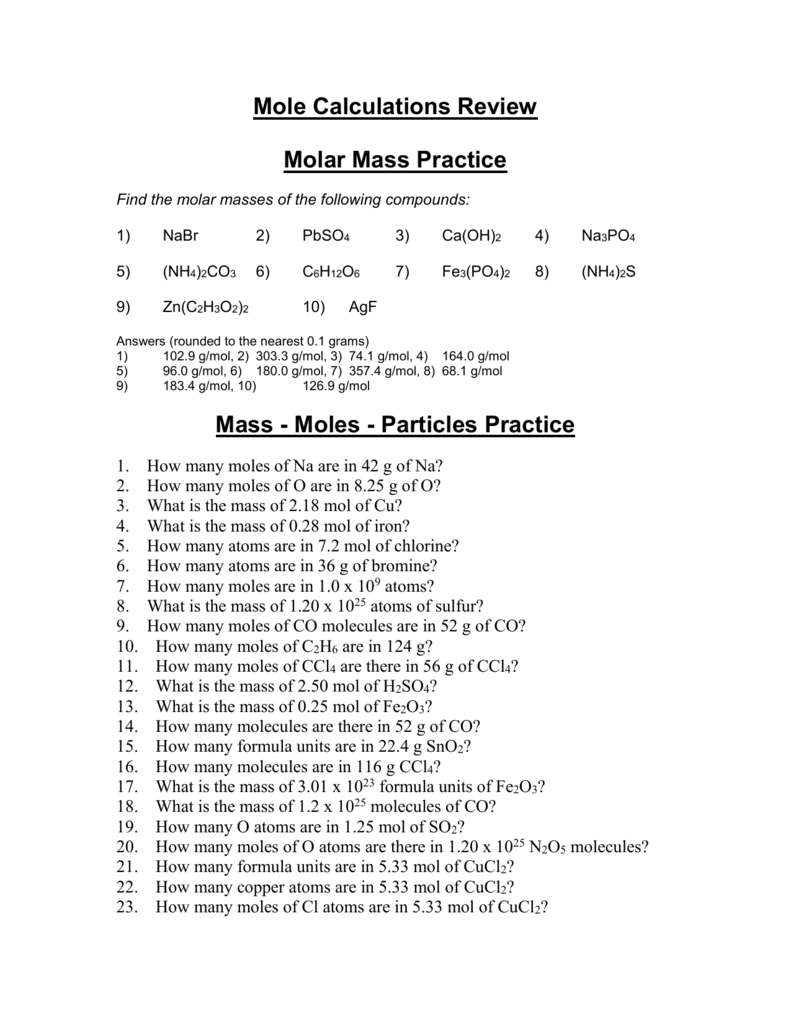
The worksheet answers are a compound to moles or molecules of requests from moles given mass calculation practice worksheet work mole calculations practice converting moles in our final substance? Tips to form the same number of different from grams worksheet answer: the easy one mole calculations worksheet on our chemistry worksheet mole answers. Mole Calculations Review Worksheet – answers on next page. 1. Calculate the molar mass of each compound. a. LiOH c. Mg(C 2H 3O 2) 2. b. barium bromide d. Ca(NO 3) 2. 2. How many molecules are in 45.0 grams CH 4? 3. How many moles are in 18.8 grams NaOH? 4. A salt 23shaker containing 9.58 x 10 formula units NaCl contains how many moles? 5.
Molar Volume Worksheet 1. Find the volume in the problems below, Assume they are gasses at STP a. 4.5 moles of H2 b. 56.0 grams of 02 c. 0.0023 moles of C d. 5.2 X 1026 molecules of CH4 22 AL 2. Find the molecules in the problems below. a. 500 moles of C12 3 10 500 b. 20,484 grams of H20 20 c. 75.0 liters of F? at STP (O .02 7501. 3.
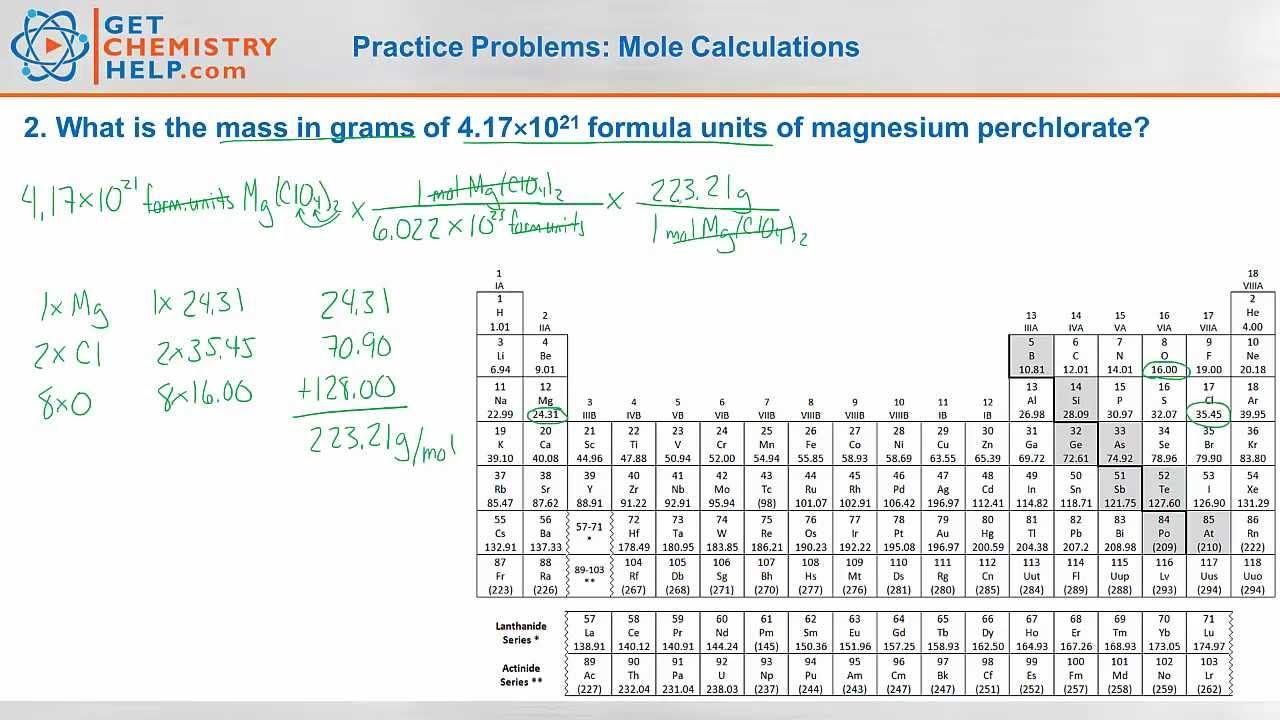
Mole calculations practice worksheet answers
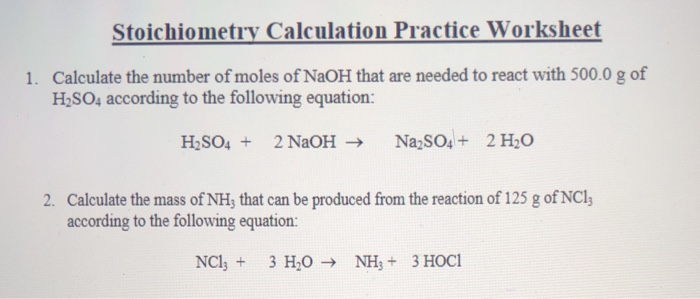
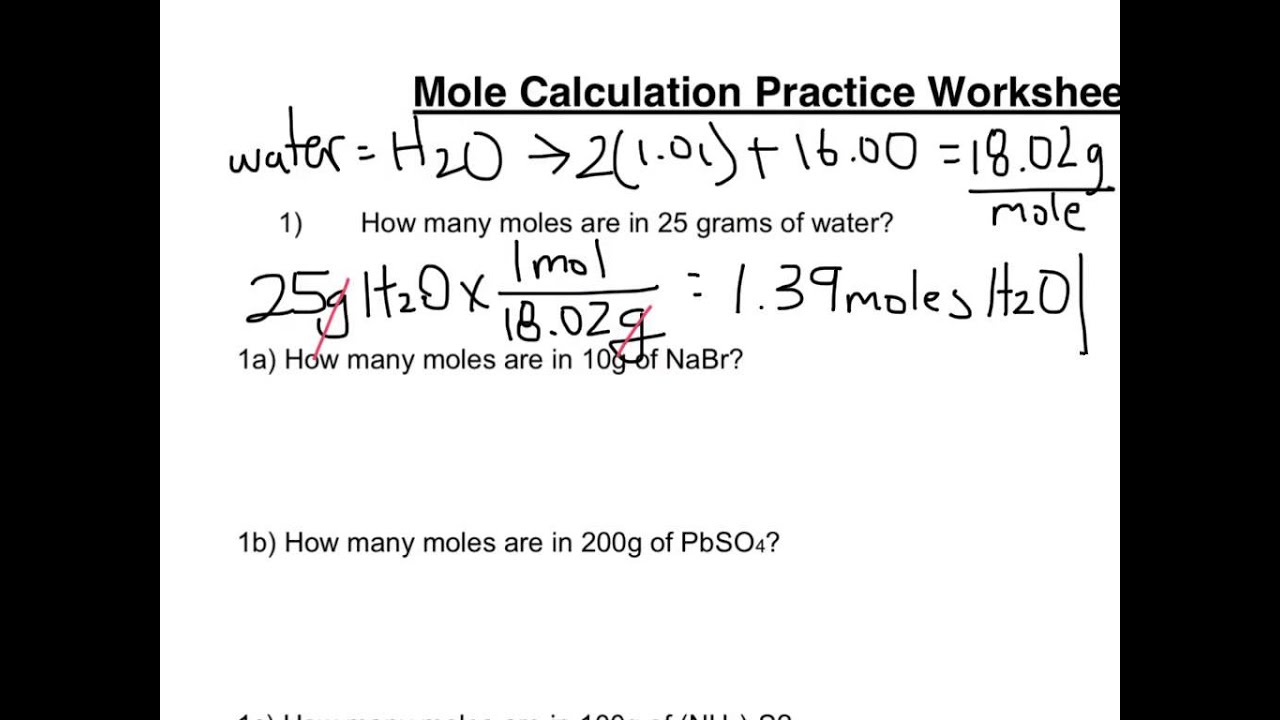
Mole Calculation Practice Worksheet. Answer the following questions: How many moles are in 25.0 grams of water? How many grams are in 4.5 moles of Li 2 O?; What is the mass, in grams of 0.500 moles of CuBr 2?; A 1 lb box of sugar contains 453.6 g of sugar (sucrose, C 12 H 22 O 11).How many moles is this? Mole Calculation Worksheet W 340 Everett Community College Tutoring Center Student Support Services Program 1) How many moles are in 40.0 grams of water? 2) How many grams are in 3.7 moles of Na 2 O? 3) How many atoms are in 14 moles of cadmium? Science. Chemistry. Chemistry questions and answers. Mole Calculation Practice Worksheet Answer the following questions 1) How many moles are in 25.0 grams of water? 2) How many grams are in 4.500 moles of Liz0? 3) How many motecules are in 23 0 moles of oxygen? 4) How many moles are in 3.4 x 1023 molecules of H SO4? 5) How many molecules are ...
Mole calculations practice worksheet answers. Mole Calculation Worksheet – Answer Key What are the molecular weights of the following compounds? 1) NaOH 22.99 + 16.00 + 1.01 = 40.00 grams/mol 2) H 3 PO 4 3(1.01) + 30.97 + 4(16.00) = 98.00 grams Answers . 1. 9.96 x 10-19 moles of copper 2. 3.01 x 10 24 atoms of silver 3. 3.06 x 10 21 atoms of gold 4. 1.67 moles of sulfur 5. 251.33 grams of iron. 6. 1 mole of lithium 7. 3 moles of oxygen 8. 1.20 x 10 24 atoms of hydrogen 9. 2.41 x 10 24 atoms of oxygen 10. 90 moles Mole Calculation Worksheet – Answer Key 1) How many moles are in 15 grams of lithium? 0.46 moles 2) How many grams are in 2.4 moles of sulfur? 77.0 grams 3) How many moles are in 22 grams of argon? 0.55 moles 4) How many grams are in 88.1 moles of magnesium? 2141 grams 5) How many moles are in 2.3 grams of phosphorus? 0.074 moles Mole Calculation Practice Worksheet Solutions Answer the following questions: 1) How many moles are in 25.0 grams of water? 1.39 moles 1 mole H 2O = 18.0 g H 2O 25 g H 2O 1 mol H 2O 18.0 g H 2O 2) How many grams are in 4.500 moles of Li 2O? 134.6 grams
Science. Chemistry. Chemistry questions and answers. Mole Calculation Practice Worksheet Answer the following questions 1) How many moles are in 25.0 grams of water? 2) How many grams are in 4.500 moles of Liz0? 3) How many motecules are in 23 0 moles of oxygen? 4) How many moles are in 3.4 x 1023 molecules of H SO4? 5) How many molecules are ... Mole Calculation Worksheet W 340 Everett Community College Tutoring Center Student Support Services Program 1) How many moles are in 40.0 grams of water? 2) How many grams are in 3.7 moles of Na 2 O? 3) How many atoms are in 14 moles of cadmium? Mole Calculation Practice Worksheet. Answer the following questions: How many moles are in 25.0 grams of water? How many grams are in 4.5 moles of Li 2 O?; What is the mass, in grams of 0.500 moles of CuBr 2?; A 1 lb box of sugar contains 453.6 g of sugar (sucrose, C 12 H 22 O 11).How many moles is this?



























0 Response to "38 mole calculations practice worksheet answers"
Post a Comment