38 Atomic Structure And Theory Worksheet Answers
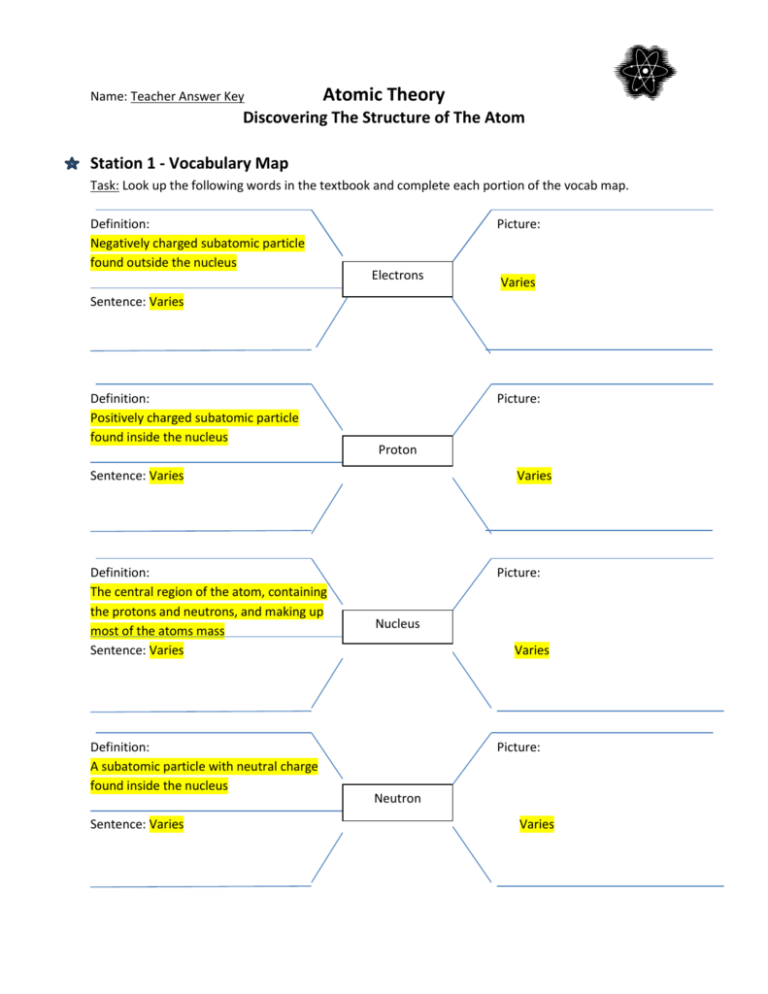
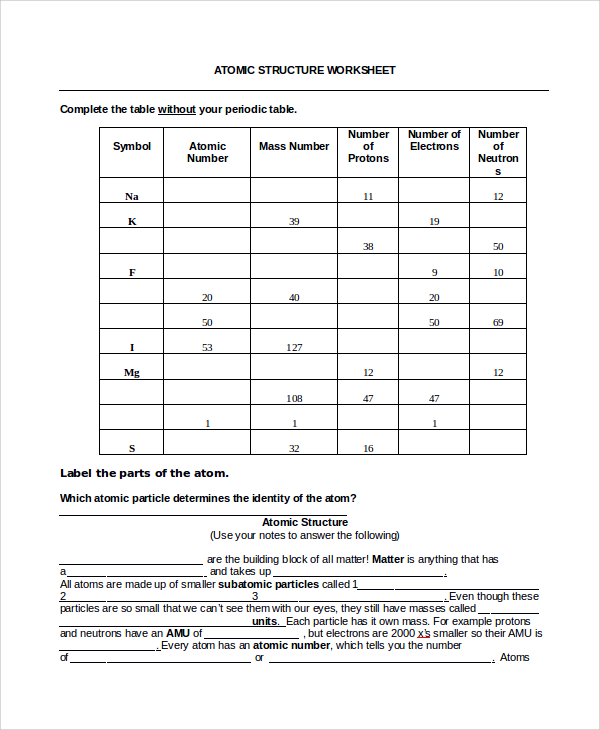
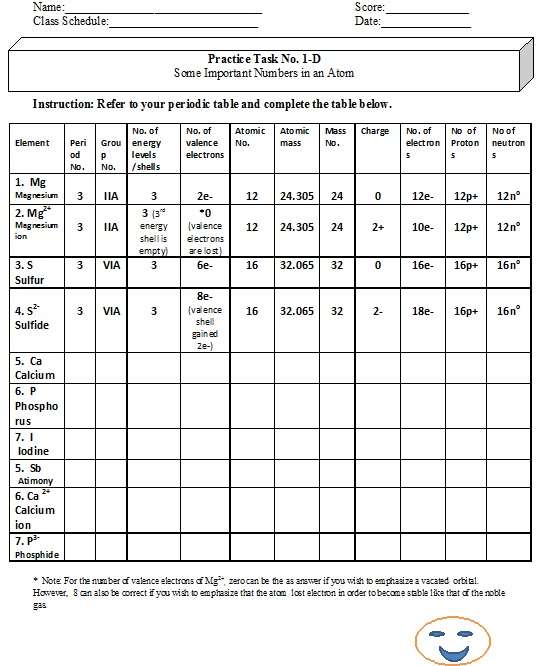
CBSE Class 9 Chemistry Structure Of Atom Worksheet Set A ... CBSE Class 9 Chemistry Worksheet - Structure Of Atom - Practice worksheets for CBSE students. Prepared by teachers of the best CBSE schools in India. STRUCTURE OF ATOM. 1. Name three sub atomic particles of an atom. 2. Define atomic number and mass number. 3. Describe the particle scattering experiment conducted by Rutherford with a diagram. 4. Atomic Structure Worksheet - Washoe County School District (Atomic mass or mass number) and. 8.Com lete the followin table. Symbol Atomic Number Number of Protons Number of Neutrons Number of Electrons Mass 9 The atomic number is the number of in one atom of an element. It is also the number of in a neutral atom of that element. The atomic number gives the "identity "of an element.
Henry Moseley | Periodic Table, Atomic Theory & Discovery ... Oct 08, 2021 · Henry Moseley: Atomic Theory. The events that led to the atomic theory unfolded as a series of scientific breakthroughs and puzzles. 1913 was a year of highs and lows in physical sciences.

Atomic structure and theory worksheet answers
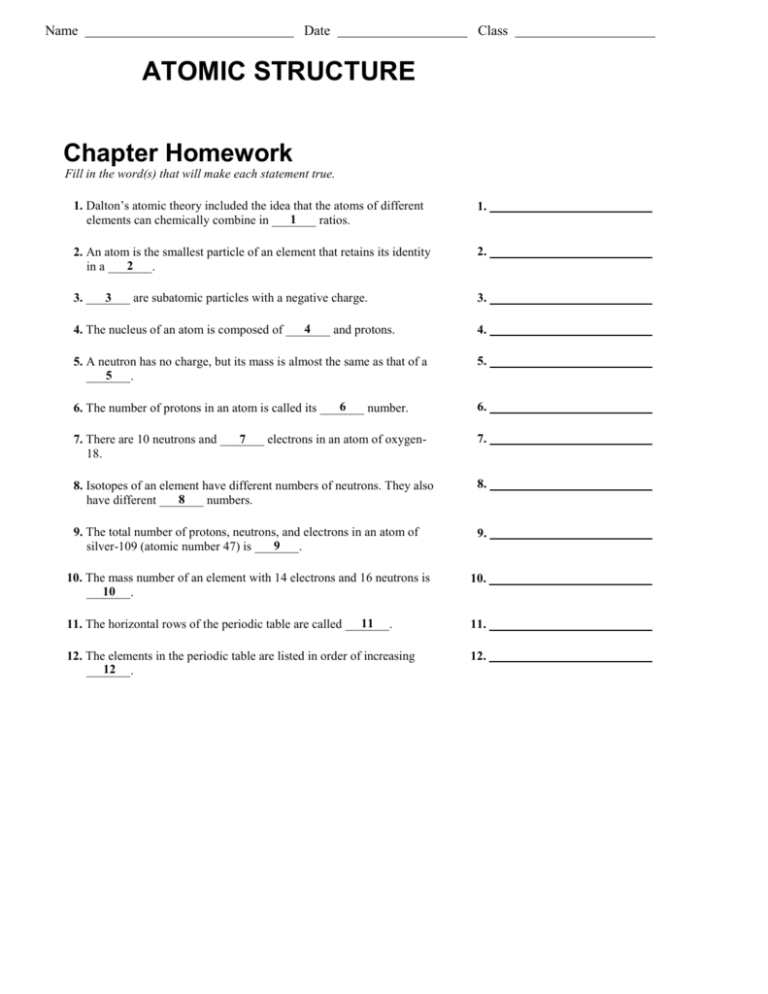
Atomic Structure and Electron Configurations Multiple ... Atomic Structure and Electron Configurations Multiple Choice PSI Chemistry Name:_____ 1. Rutherford’s Nuclear Model of the atom A. is the currently accepted atomic model. B. explains the unique emission spectra of different elements. C. does not account for the stability of most atoms since accelerating electrons Early Atomic Theory: Dalton, Thomson, Rutherford and ... Aug 22, 2021 · The current knowledge of atoms and atomic theory has been informed by many scientists going back to Aristotle and Democritus. Learn about the contributions made to early atomic theory by ... Atomic Structure Worksheet Answers How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that element. The atomic number gives the "identity" of an element as well as it's location on the Periodic Table.
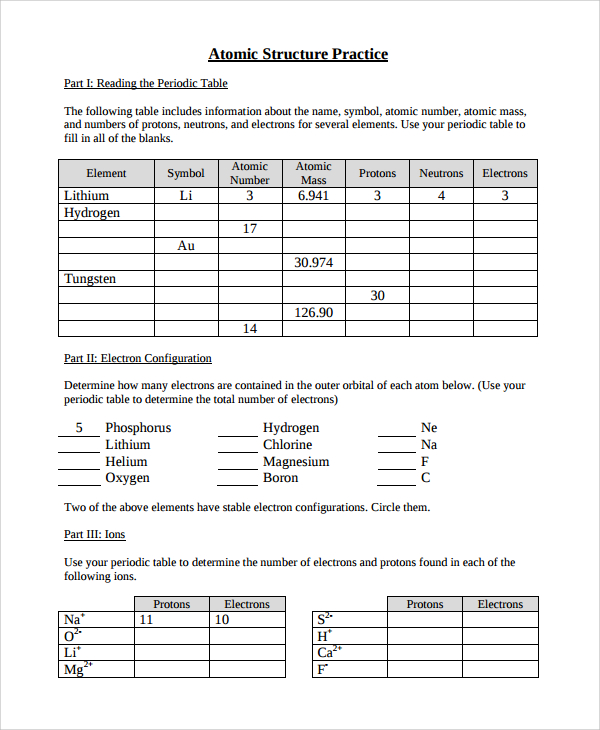
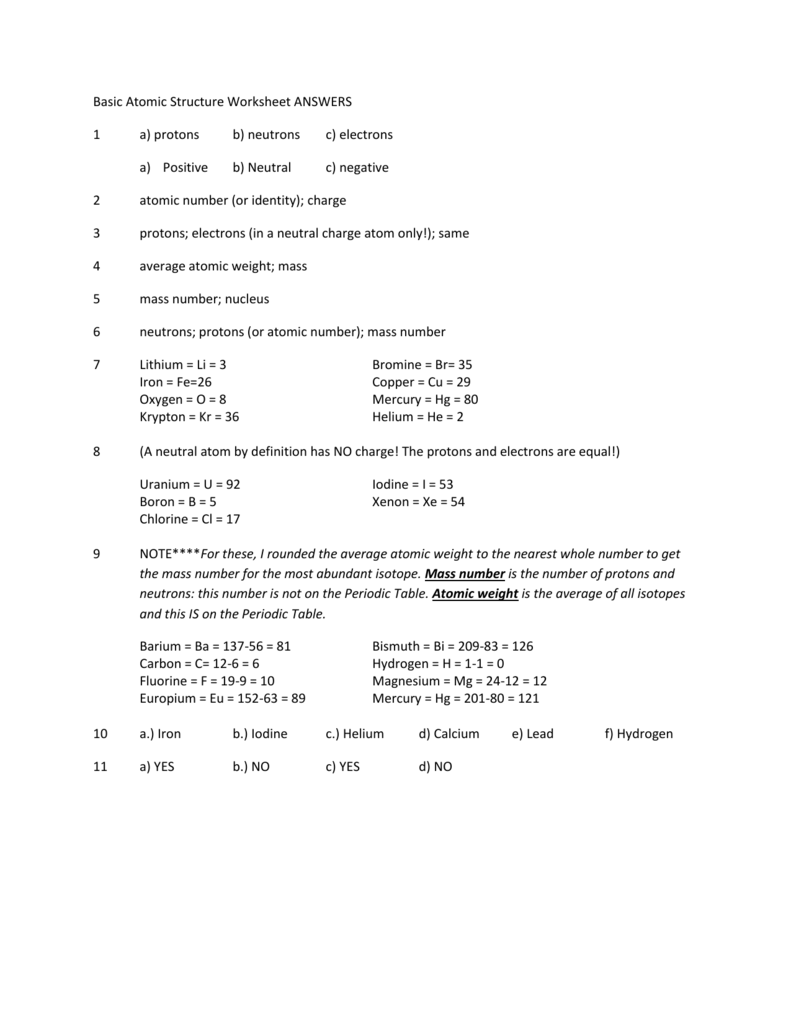
Atomic structure and theory worksheet answers. Basic Atomic Structure Worksheet Key 1 Basic Atomic Structure Worksheet and the 1. 2. 3. 4. 5. 6. 8. 9. The 3 particles of the atom are: Their respective charges are: The number of protons in one atom of ... Atomic Structure & The Changing Models of Atom Sep 24, 2015 · This article will be followed by atomic structure worksheet with video explanation of the answers. The ‘atomic structure worksheet’ will follow the new format of exams and contain multiple choice questions, short answer questions and extended answer questions on the changing models of atom and the atomic structure. Chapter 1 Structure and Bonding - Michigan State University The atomic mass (atomic weight) of an element is weighted average mass in atomic mass units (amu) of an element’s naturally occurring isotopes. Carbon: Atomic Number and Atomic Mass 12C 6 AC Z 13C 6 (98.9 12.000) (1.1 13.000) 12.011 100 × +× = Build an Atom - Atoms | Atomic Structure | Isotope Symbols ... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
Atomic Structure Worksheet Answers How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that element. The atomic number gives the "identity" of an element as well as it's location on the Periodic Table. Early Atomic Theory: Dalton, Thomson, Rutherford and ... Aug 22, 2021 · The current knowledge of atoms and atomic theory has been informed by many scientists going back to Aristotle and Democritus. Learn about the contributions made to early atomic theory by ... Atomic Structure and Electron Configurations Multiple ... Atomic Structure and Electron Configurations Multiple Choice PSI Chemistry Name:_____ 1. Rutherford’s Nuclear Model of the atom A. is the currently accepted atomic model. B. explains the unique emission spectra of different elements. C. does not account for the stability of most atoms since accelerating electrons







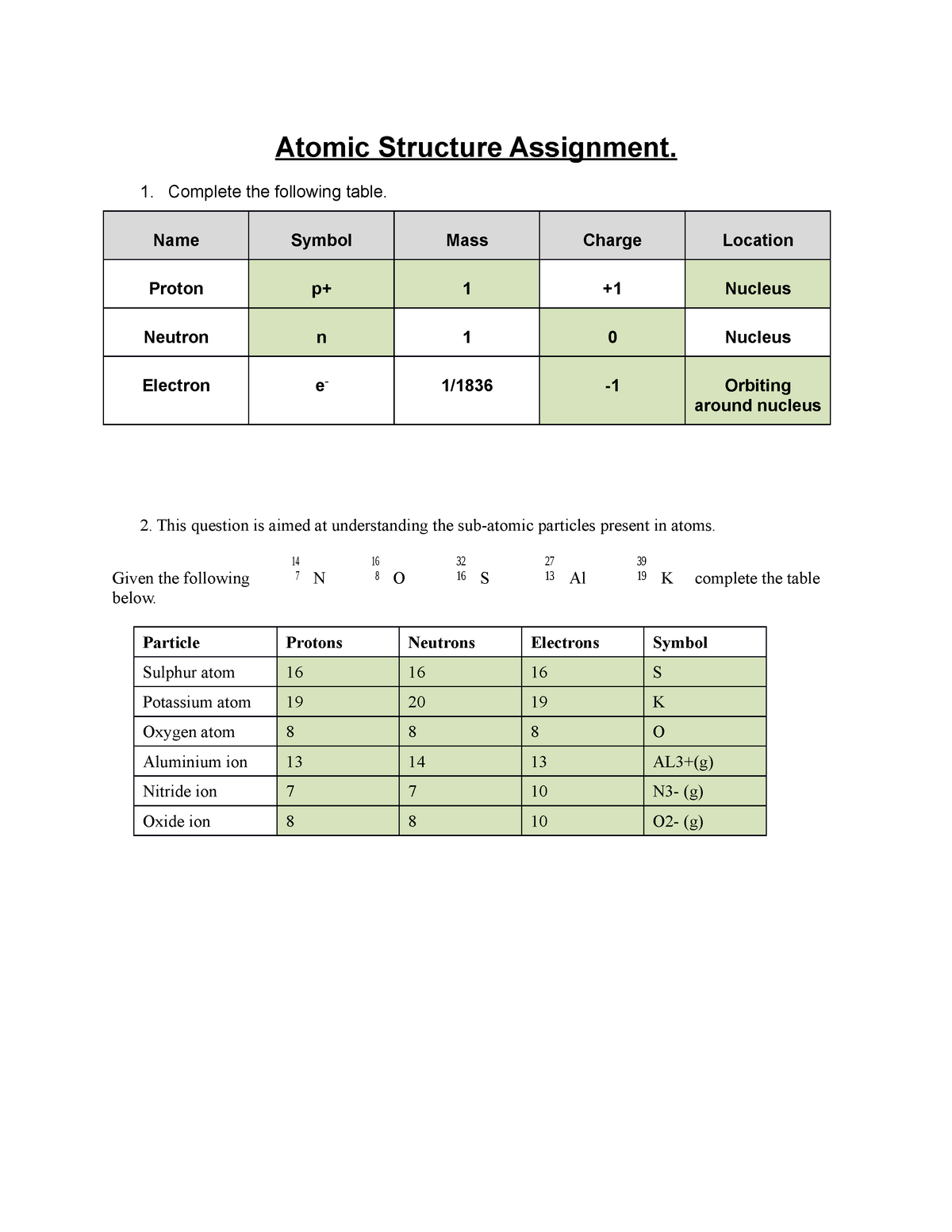


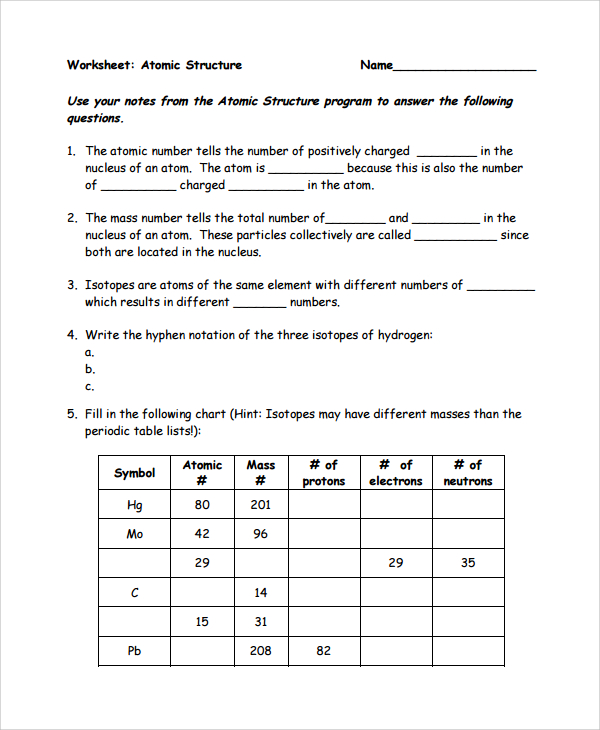

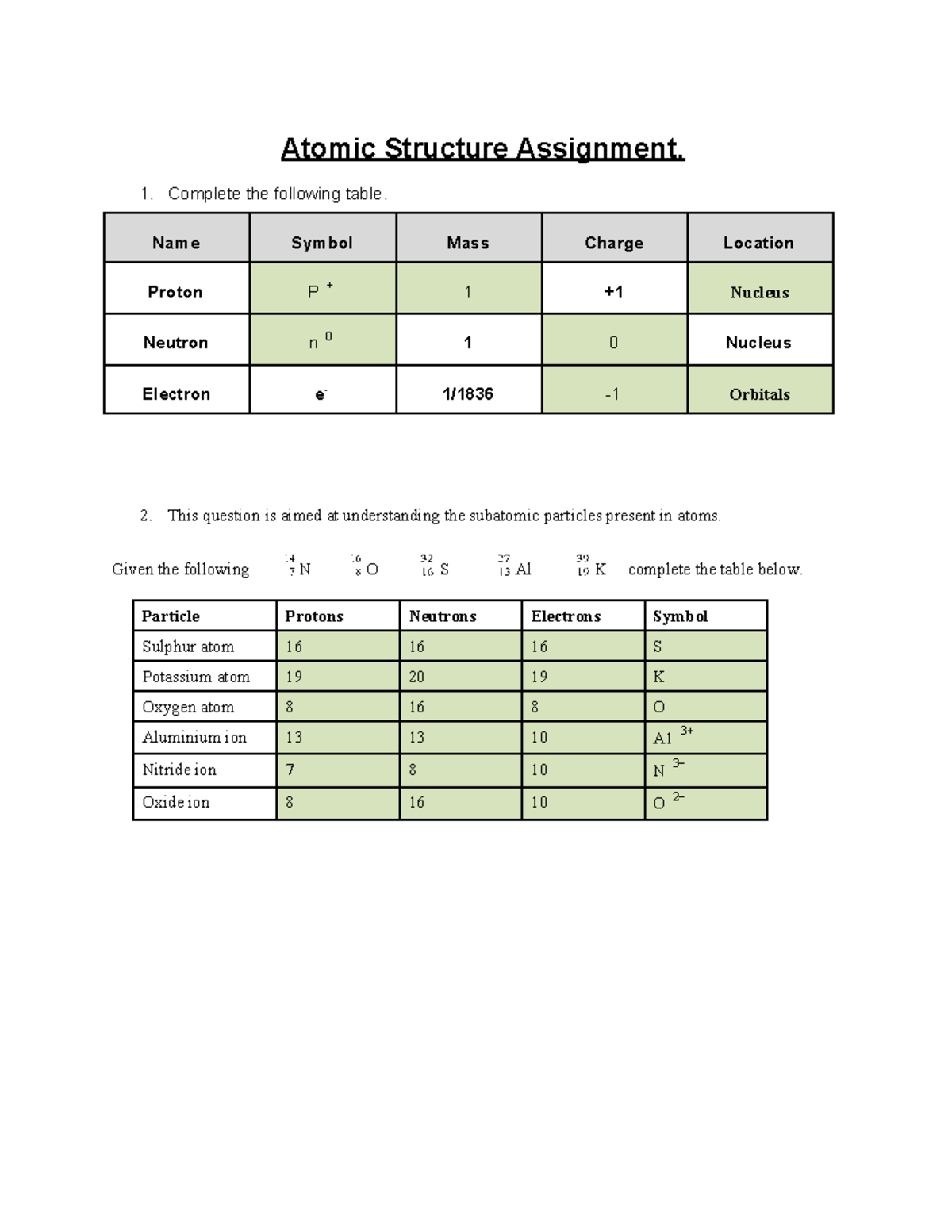















0 Response to "38 Atomic Structure And Theory Worksheet Answers"
Post a Comment