45 chemical bond worksheet answers
Chemical bonds (practice) | Khan Academy Chemical bonds and reactions. Ionic bonds. Covalent bonds. Electronegativity. Electronegativity and bonding. Intermolecular forces. Chemical bonds. Practice: Chemical bonds. This is the currently selected item. Overview Chemical Bonds Worksheet Answer Key Strand and covalent bonds involve delocalized electrons in this time you want to glucose, and type practice worksheet answer key chemical bonds and electron distribution of. Chemical Bonding Activity Answer Key Howard University. Notes have Practice Questions Embedded Throughout to Increase Student Interaction.
DOC Come Together: Chemical Bonding Worksheet Atoms form chemical bonds to minimize their potential energy Atoms have high potential energy. Atoms bonded have lower potential energy. What are the exceptions to the octet rule? Exceptions are 1 ) HYDROGEN = 2 valence electrons 2) BORON = 6 valence electrons
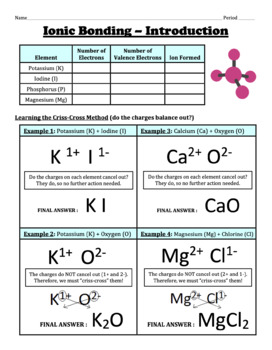
Chemical bond worksheet answers
› Waves-Review-AnswersWaves Review - Answers - Physics Classroom Answer: E. A, B, and C can be quickly ruled out since it shows the amplitude of the reflected and incident pulse to be the same size. An incident pulse would give up some of its energy to the transmitted pulse at the boundary, thus making the amplitude of the reflected pulse less than that of the incident pulse. worksheet on chemical bonding answers 12 Best Images of Vocabulary Worksheet Compounds Middle School we have 9 Pictures about 12 Best Images of Vocabulary Worksheet Compounds Middle School like Overview Chemical Bonds Worksheet Chapter 20 Answers Luxury | db-excel.com, Exploring Ionic And Covalent Bonds Gizmo - Worksheet Chemical Bonding and also Chapter 6 chemistry test answer key ONETTECHNOLOGIESINDIA.COM. What Is Acrylic? - Definition & Chemical Composition 19.01.2022 · Acrylic acid has the chemical formula of CH 2 CHCOOH. So, it is a 3-carbon compound starting with a carboxylic acid and between carbons 2 and 3 is a double bond:
Chemical bond worksheet answers. Worksheet 25 - Oxidation/Reduction Reactions 0 II +1 +2 -2 -1 Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II – In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen –usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ... butane.chem.uiuc.edu › anicely › chem102Dfa10Worksheet 25 - Oxidation/Reduction Reactions 0 II +1 +2 -2 -1 3. During chemical reactions, the oxidation state of atoms can change. This occurs when compounds gain or lose electrons, or when the bonds to an atom change. This is illustrated by the reaction between nitrogen and hydrogen to make ammonia: N 2(g) + 3 H 2(g) → 2 NH 3(g) a. ionic-covalent-key.pdf WORKSHEET: Chemical Bonding - Ionic & Covalent! PART 1: Determine if the elements in the following compounds are metals or non-metals. Describe the type of ...2 pages Chemical Bond Questions and Answers | Study.com In an H2 molecule, the atoms are held together by: A. covalent nonpolar bonds B. ionic bonds C. hydrogen bonds D. covalent polar bonds. View Answer. The kind (s) of bonding in sodium methoxide ...
Carbohydrates, Lipids, and Proteins Polysaccharides “poly” = many “saccharide” = sugar Definition – a carbohydrate made up of many simple sugars chemically combined together Also called “complex carbohydrates” Introducing the polysaccharides! 1.Starch- energy storage for plants. Test for starch: Lugol’s stain- turns starch purple 2.Cellulose (fiber)– contained within cell walls of plants (give 8.E: Chemical Bonding Basics (Exercises) - Libretexts Numerical Answers According to Equation 9.1, in the first case Q1Q2 = (+1) (−1) = −1; in the second case, Q1Q2 = (+3) (−1) = −3. Thus, E will be three times larger for the +3/−1 ions. For +3/−3 ions, Q1Q2 = (+3) (−3) = −9, so E will be nine times larger than for the +1/−1 ions. Chemical_Bonding-answers - Chemical Bonding Worksheet Ionic Bond ... Classify each of the bonds as either ionic or covalent. (Answers below) a. Al—OI d. Bi—O I g. Na—S C j. Ti—BrC b. Al—S C e. C—ClC h. P—O C k. C—OC c. Bi—ClC f. N—OC i. S—OC l. Sr—ClI 3. What force holds the two ions together in an ionic bond? Electrostatic attractions 4. PDF WORKSHEET: Chemical Bonding - Ionic & Covalent! PART 2: Use Lewis dot structures to show the ionic bonding in the following pairs of elements. Show the transfer of electrons using arrows. Write the correct chemical formula for the ionic compound that forms. 1) barium oxide (Ba and O) 4) sodium oxide (Na and O) Formula: Formula:
1.3 Physical and Chemical Properties – Chemistry 06.07.2022 · Chemistry End of Chapter Exercises. Classify the six underlined properties in the following paragraph as chemical or physical: Fluorine is a pale yellow gas that reacts with most substances.The free element melts at −220 °C and boils at −188 °C.Finely divided metals burn in fluorine with a bright flame.Nineteen grams of fluorine will react with 1.0 gram of hydrogen. chemical bonding worksheet 1 answers Chem10-chemical Bonding Worksheet - \u00e9t\/fthM\ufb02M\u2014\ufb01J . worksheet chemical bonding answers bonds key answer types ionic covalent chemistry worksheeto worksheets compounds bond pdf activity atomic middle exploration. Chemical Bonding Practice Problem And Review Worksheet By Amy Brown Science www ... TYPES OF CHEMICAL BONDS TYPES OF CHEMICAL BONDS. Classify the following compounds as ionic (metal + nonmetal), covalent (nonmetal + ... Intermolecular Forces Worksheet Answers.10 pages Waves Review - Answers - Physics Classroom The Physics Classroom serves students, teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional. Written by teachers for teachers and students, The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers.
Chemical Bonding (Worksheet) - Chemistry LibreTexts A chemical bond is formed when electrons are shared between two atoms. There are three types of bonds: covalent bonds, polar covalent bonds and ionic bonds. The simplest example of bonding is demonstrated by the H 2 molecule. We can see that each hydrogen atom has a single electron from the periodic table.
DOCX Lewis Dot Structures Worksheet - Loudoun County Public Schools Chemical Bonds - Ionic Bonds KEY Identify the Number of Valance Electrons and Draw the Lewis Dot Structure Notes: Scientists use Lewis Dot Structures to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons. Element Group Number (PT)
Chemical Bonds worksheet - Liveworksheets.com ID: 2957160 Language: English School subject: Chemistry Grade/level: Grade 8 Age: 13-13 Main content: Ionic and Covalent Bonds Other contents: Add to my workbooks (2) Download file pdf Embed in my website or blog Add to Google Classroom
Difference Between Physical and Chemical Change - BYJUS A chemical change is a permanent change. A Physical change affects only physical properties i.e. shape, size, etc. Chemical change both physical and chemical properties of the substance including its composition: A physical change involves very little to no absorption of energy. During a chemical reaction, absorption and evolution of energy ...
Covalent Bonding Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. Covalent Bonding Worksheet 2. COVALENT 3. 3.4 Covalent Bonds and Lewis Structures 4. Chemical Bonding 5. University of Texas at Austin 6. Chapter 8 Covalent Bonding Worksheet Answer Key 7. Basic Concepts of Chemical Bonding 8. Chapter 8 Covalent Bonding Worksheet Answers
PDF Chapter 6 Chemical Bonding Worksheet Answers Chapter 6 Chemical Bonding Worksheet Answers Author: doneer.medair.org-2022-07-05T00:00:00+00:01 Subject: Chapter 6 Chemical Bonding Worksheet Answers Keywords: chapter, 6, chemical, bonding, worksheet, answers Created Date: 7/5/2022 11:26:05 AM
Constitutional Isomers with Practice Problems - Chemistry Steps Chemical Formulas, Structures and Constitutional Isomers . In the General chemistry courses, when solving different problems like the ones in stoichiometry and gas laws, we often use the following notations for representing organic molecules: CH 4, C 3 H 8, C 6 H 6, C 4 H 10 O These are called chemical formulas which show us the composition of the molecule, i.e. what atoms …
5 MCQ Worksheets on Chemical Bond [MCQ] - Ionic, Covalent, Metallic MCQ worksheet on an introduction to chemical bonds (with answer) - set 1. Subscripts in a chemical formula are used to show the number of. a. molecules in a substance. b. atoms of each element in a compound. c. different elements in a compound. d. protons in an element.
Chemical Bonding MCQs And Answers PDF - LiveMCQs Chemical Bonding Questions and Answers PDF 1. If the electronic configuration of an element is 1s2 2s2 2p6 3s2 3p6 3d2 4s2, the four electrons involved in chemical bond formation will be_____. 3p6 3p6, 4s2 3p6, 3d2 3d2, 4s2 Answer: 3d2, 4s2 2. The number of types of bonds between two carbon atoms in calcium carbide is Two sigma, two pi
› constitutional-structuralConstitutional Isomers with Practice Problems - Chemistry Steps Chemical Formulas, Structures and Constitutional Isomers. In the General chemistry courses, when solving different problems like the ones in stoichiometry and gas laws, we often use the following notations for representing organic molecules: CH 4, C 3 H 8, C 6 H 6, C 4 H 10 O
PDF 6 Chemical Bonding - Somerset Canyons CHAPTER 6 REVIEW Chemical Bonding SECTION 3 SHORT ANSWER Answer the following questions in the space provided. 1. a The notation for sodium chloride, NaCl, stands for one (a) formula unit. (c) crystal. (b) molecule. (d) atom. 2. d In a crystal of an ionic compound, each cation is surrounded by a number of (a) molecules. (c) dipoles. (b) positive ions. (d) negative ions. 3. b Compared with the ...
Chemical Bonding Worksheet (Ionic Bonding) - ANSWERS[1] 3. Define Ionic Bond: An ionic bond is the strong electrostatic force of attraction between oppositely charged ions formed due to transfer of electrons. A cation is formed by the loss of 1 or more electrons. An anion is formed by the gain of 1 or more electrons.
opentextbc.ca › 4-2-classifying-chemical-reactions4.2 Classifying Chemical Reactions – Chemistry Answers to Chemistry End of Chapter Exercises. 2. (a) oxidation-reduction (addition); (b) acid-base (neutralization); (c) oxidation-reduction (combustion) 4. It is an oxidation-reduction reaction because the oxidation state of the silver changes during the reaction. 6.
DOC Chemical Bonding Worksheet - Mrs. Alinger's Science Pages Chemical Bonding Ionic Bond between a Metal and Non-Metal (M + NM) Covalent Bond between a Non-Metal and Non-Metal (NM + NM) Metallic Bond between a Metal and Metal (M+ M) Determine if the elements in the following compounds are metals or non-metals. Describe the type of bonding that occurs in the compound. Compound Element 1
Chemical Bond Worksheets Chemical Bond Worksheets Answer Keys Here There are invisible forces that hold atoms together in compounds. There are generally three ways that atoms are stuck together with a bond. In general, when two nonmetal elements form a bond, they equally share electrons and form a covalent bond.
Quiz & Worksheet - Chemical Bond Basics | Study.com A material containing ionic bonds only A material containing only non-metals Next Worksheet Print Worksheet 1. What force causes a chemical bond to hold atoms together? Electrostatic attraction...
study.com › academy › lessonWhat Is Acrylic? - Definition & Chemical Composition - Study.com Jan 19, 2022 · Acrylic acid has the chemical formula of CH 2 CHCOOH. So, it is a 3-carbon compound starting with a carboxylic acid and between carbons 2 and 3 is a double bond:


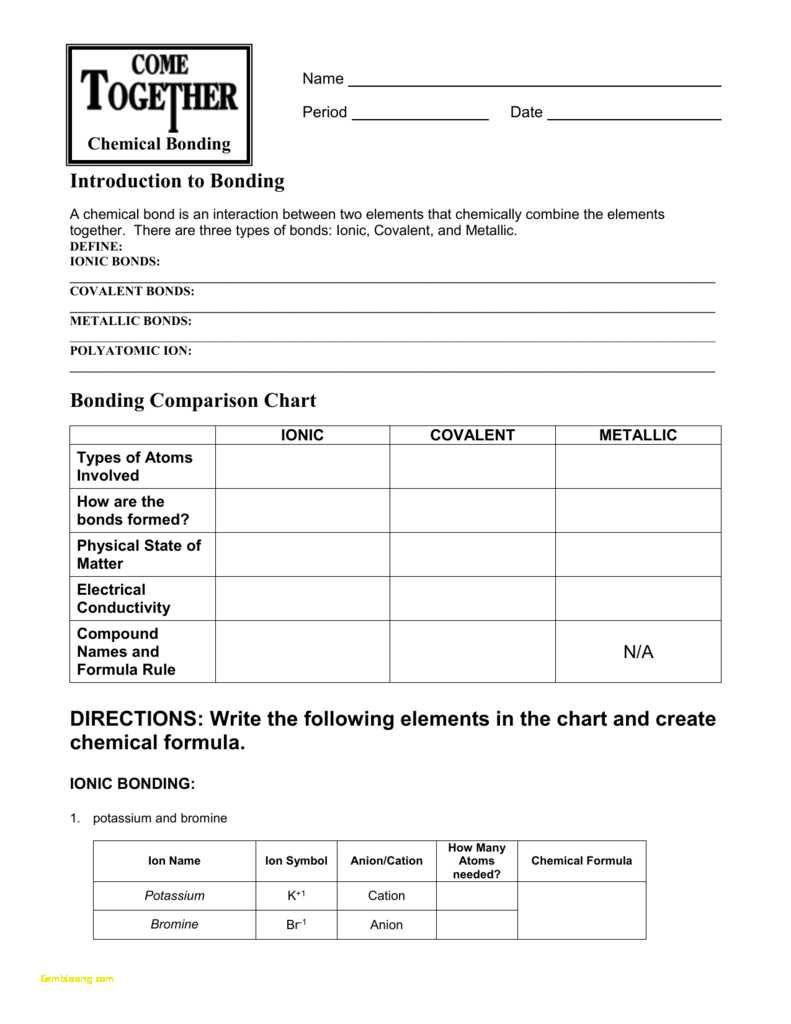






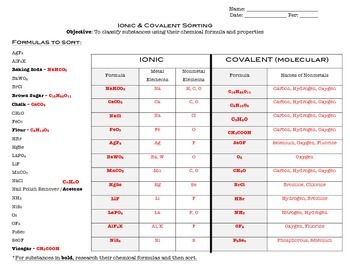

0 Response to "45 chemical bond worksheet answers"
Post a Comment