44 more average atomic mass worksheet answers
PDF Atomic Mass 37. Calculate the average atomic mass of chlorine. 2. Copper has two isotopes. Copper-63, which has an atomic mass of 62.93 u and copper-65, which has an atomic mass of 64.93 u. In any sample of copper atoms, 69.1% will be copper-63 and 30.9% will be copper-65. Calculate the average atomic mass of naturally occurring copper. 3. One atom has 20 ... Average Atomic Mass Worksheet.docx - Course Hero Average Atomic Mass Worksheet: show all work. 1) Rubidium is a soft, silvery-white metal that has two common isotopes,85Rb and87Rb. If the abundance of85Rb is 72.2% and the abundance of87Rb is 27.8%, what is the average atomic mass of rubidium? 2) Uranium is used in nuclear reactors and is a rare element on earth. Uranium has three common isotopes.
Average Atomic Mass and Percent Abundance Worksheet 2 ... Average Atomic Mass and Percent Abundance Worksheet 2 and KEY - Free download ... Since the carbon-12 isotope is more abundant, its mass is weighted more in ...

More average atomic mass worksheet answers
Atomic Mass Worksheet Key. Average Atomic Mass Worksheet: show all work. 1) Rubidium is a soft, ... the abundance of 238U is 99.28%, what is the average atomic mass of uranium? Practice - Average Atomic Mass Worksheet 1.1 - Answer Key Bundle - Average Atomic Mass Practice Worksheet 1.1 + Answer Key Save 5% off regularly priced items with this bundle!!The Chemistry Teacher WebsiteThe Chemistry Teacher on YouTube 2 Products $ 2.35 $ 2.48 Save $ 0.13 View Bundle Bundle - Average Atomic Mass Practice Worksheets 1.0, 1.1, & 2.0 + Answer Keys Average Atomic Mass Practice - fair Part I. Average Atomic Mass of Zirconium. A device known as a mass spectrometer can be used to determine the relative abundance of the isotopes of elements.
More average atomic mass worksheet answers. Average Atomic Mass Flashcards | Quizlet It's already practical because it's a decimal so it's more approximate. 12. Propose a possible way to calculate the average atomic mass of 100 magnesium atoms. Your answer may include a mathematical equation, but it is not required. Add all of the masses together and divide by how many masses there are. Mary's method. Calculating Average Atomic Mass Worksheet Answers (PDF) - voice.edu Average Atomic Mass Worksheet Answers It will not acknowledge many grow old as we tell before. You can complete it even though pretend something else at house and even in your workplace. correspondingly easy! So, are you question? Just exercise just what we find the money for under as well as evaluation Calculating Mathematics of Satellite Motion - Physics Classroom where T is the period of the satellite, R is the average radius of orbit for the satellite (distance from center of central planet), and G is 6.673 x 10-11 N•m 2 /kg 2. There is an important concept evident in all three of these equations - the period, speed and the acceleration of an orbiting satellite are not dependent upon the mass of the satellite. Quiz & Worksheet - Pharmacokinetics | Study.com This quiz and worksheet will assess your grasp of pharmacokinetics and the functions that makeup this process. Quiz question cover the metabolism and absorption of medications, and the primary ...
Oxygen - Element information, properties and uses | Periodic Table Relative atomic mass The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Isotopes Atoms of the same element with different numbers of neutrons. CAS number The Chemical Abstracts Service registry number is … DOC Chemistry Worksheet - Humble Independent School District More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. Build an Atom - Atoms | Atomic Structure | Isotope Symbols Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Then play a game to test your ideas! Skip to Main Content Calculating Average Atomic Mass Worksheet The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93 amu. Calculate the average atomic mass of copper. 0.692( ...
PDF More Average Atomic Mass - S.W.H.S CHEMISTRY More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. 2.3: Calculating Atomic Masses (Problems) - Chemistry LibreTexts PROBLEM 2.3. 4. Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments. Mole Concept- Formula, Explanations, Examples, Related … Mole Concept- A mole is defined as the amount of a substance that contains exactly the Avogadro number of ‘elementary entities’ of the given substance. The Avogadro number is represented by NA. The Mole Concept is a Convenient Method of Expressing the Amount of a Substance. To Learn more about the Mole Concept with Formulae and Examples with Videos … PDF More Average Atomic Mass - S.W.H.S CHEMISTRY More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 178.55 amu 2.
PDF NAME Average Atomic Mass Worksheet: show all work. and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 35.46 amu 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63 ...
Average Atomic Mass - Mr. Sault's Classroom a. Which isotope has an atomic mass closest to the average atomic mass listed on the periodic table? 24.mg b. Give a mathematical reason for your answer to part a. 24 Mg is the most common isotope and is thus most heavily "weighted" in the equation for average atomic mass. 17. Boron has two naturally occurring isotopes: boron-10 and boron-11.
Worksheets | Telecom Fiji Worksheet 2 - Fill in the Blanks, Mass Media, Library & Dictionary & Composition Worksheet 3 - Short Story, Poetry and Picture Composition Worksheet 4 - Comprehension, Parts of Speech, Sentence Completion & Vocabulary
Average Atomic Mass Practice Problems Quiz - Quizizz 6 Questions Show answers Question 1 900 seconds Q. 24.1% of all the isotopes of a an element have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. What is the average mass of this element? answer choices 74.92 amu 24.97 amu 75.01 amu 74.51 amu Question 2 120 seconds Q. Calcium has three different isotopes.
Average Atomic Mass Worksheet.pdf - Name: _ Date: _ Period:... Average atomic mass = (mass x % abundance) + (mass x % abundance) + …… 100 1) Rubidium has two common isotopes, 85Rb and 87Rb. If the abundance of 85Rb is 72.2% and the abundance of 87Rb is27.8%, what is the average atomic mass of rubidium? 2) Uranium has three common isotopes.
PDF isotopic abundance practice problems - Maurer Math Using the average mass from the periodic table, calculate the abundance of each isotope.! atomic mass = mass 1 1)+ mass 2 2)+ . . . carbon 6 C 12.011 isotope % abundance mass (amu) carbon-12 99.45 12.000 carbon-14 0.55 14.003 atomic mass = (12.000 × 0.9945)+ (14.003 × 0.0055) atomic mass = (11.934)+ (0.077)= 12.011 amu
Average Atomic Mass Is it the most common isotope's mass? The heaviest mass? This activity will help answer that question. Model 1 - A Strip of Magnesium Metal. 241.
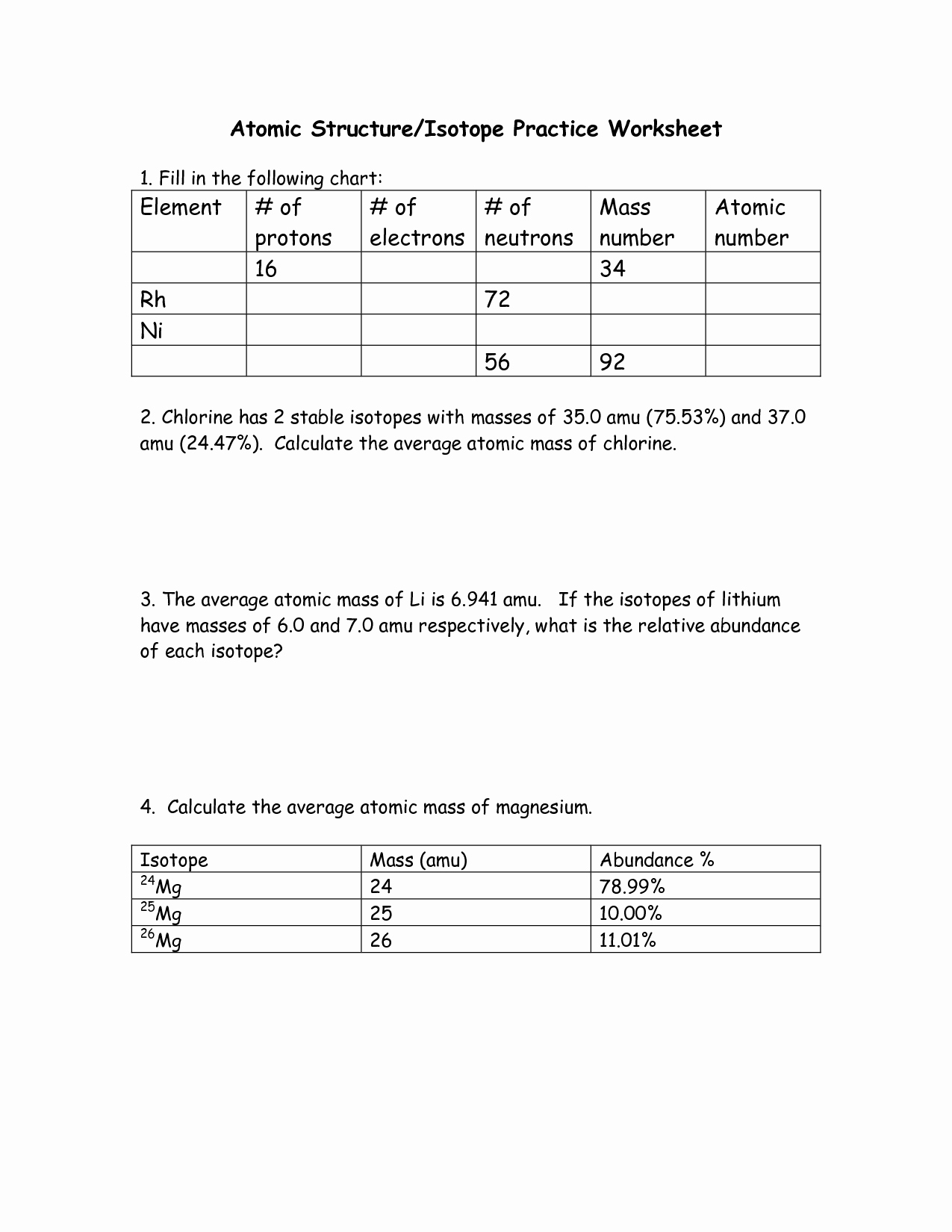
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Find the average atomic mass for Cl is 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. Find the average atomic mass for Mg if 78.99% of Mg atoms are 24Mg with a mass of 23.9850419 amu, 10.00% are 25Mg with a mass of 24.9858370 amu, and 11.01% are 26Mg with a mass of 25.9825930 amu.
Atomic Number And Atomic Mass Worksheet Answers Atomic_number_and_mass_worksheet_answers 726 Atomic Number And Mass Worksheet Answers version. Ad Download over 20000 K-8 worksheets covering math reading social studies and more. Chapter 4 3 atomic structure worksheet answer key geotwitter. The workbook you are using should have answers for those questions. Worksheet October 25 2017.
Worked Chemistry Problem Examples - ThoughtCo 22/11/2019 · Included in this list are printable pdf chemistry worksheets so you can practice problems and then check your answers. You may also browse chemistry problems according to the type of problem. You may also browse chemistry problems according to the type of problem.
Isotopes & Relative Atomic Mass (solutions, examples, videos) Molecular Mass More Lessons on Chemistry. The following diagrams show the isotopes of chlorine and how to calculate the relative atomic mass. Scroll down the page for examples and solutions. Isotopes. Isotopes are atoms of the same element that have different number of neutrons. (In order for them to be atoms of the same element, their number of protons would …
How to Find Average Atomic Mass: 8 Steps (with Pictures ... - wikiHow A molecule of water has the chemical formula H 2 O, so it contains two hydrogen (H) atoms and one oxygen (O) atom. Hydrogen has an average atomic mass of 1.00794 amu. Oxygen atoms have an average mass of 15.9994 amu. The average mass of a molecule of H 2 O equals (1.00794) (2) + 15.9994 = 18.01528 amu, equivalent to 18.01528 g/mol.
Quiz & Worksheet - Average Atomic Mass | Study.com Atomic mass Atomic number Number of electrons Number of protons Next Worksheet Print Worksheet 1. Which of the following indicates the percentage of the natural occurrence of an isotope of an...
Average Atomic Mass.docx - Average Atomic Mass Answer Key Vocabulary ... Now calculate the average mass by adding up the masses of all the cans and dividing by the total number of cans. A. What is the average mass of a can of soup? 200g [1200/6] average mass Q1) average mass of the soup is around 190g to 195g Q2) average mass of the soup = [ 250g x 2 + 100g x 3 + 1 x 4000g]/6 = 200g
PDF Atomic Structure Worksheet Answers - Columbia Public Schools 1 .999 Atomic# = Atomic Mass = # of Protons = # of Neutrons = # of Electrons = ves and Ions..... 1. What is an isotope? — 2. When an isotope is written with a word, a dash and a number, what does the number next to isotope signify? And when there is a name with two numbers, what do these number signify? Give an example of each and label. 3.
Chemistry Quizzes | Study.com 2,000,000+ Questions and Answers ... Isotopes and Average Atomic Mass . View Quiz. Predict the Formation, Charge, and Formulas of Ions ... Comparing Mass in Solutions: Quiz & Worksheet for Kids ...











0 Response to "44 more average atomic mass worksheet answers"
Post a Comment