45 atomic theory review worksheet
PDF KM 654e-20150109102424 - Columbia Public Schools How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that element. The atomic number gives the "identity" of an element as well as it's location on the Periodic Table. PDF Skills Worksheet Directed Reading A - Westerville City School District a. Atoms are small, soft particles. b. Atoms are always standing still. c.Atoms are made of a single material. d. Atoms are small particles that can be cut in half again and again. 3. We know that Democritus was right to say that all matter was made up of, atoms. So why did people ignore Democritus's ideas for such a long time? 4.
PDF Development of Atomic Theory , CHAPTER 6 REVIEW ACrlVlTY Text Reference: Section 6-90 Development of Atomic Theory Choose words from the list to fill in the blanks in the paragraphs. Word List atom atomic number Bohr Chadwick conservation of matter Dalton definite proportions electron energy level isotope Lavoisier mass number 1. multiple proportions neutron 2. nucleus

Atomic theory review worksheet
Lesson 9 Atomic Theory Review Worksheet (1) (1).docx Use the following information to determine the atomic mass of carbon. Two isotopes are known: carbon-12 (mass = 12.000 amu) and carbon-13 (mass = 13.003 amu). Their relative abundance's are 98.9% and 1.10% respectively. ( 12. 000 amu x 0.989 ) + ( 13.003 amu x 0.11 ) = 13.30 amu, 14. DOCX Saint Louis Public Schools / Homepage a) Complete the first three columns of the table for each of the proposals of atomic structure using the items in the list below. Some items may be used more than once. Some squares get two items. b) When new experimental evidence is observed that disagrees with the past understanding, there are two possible responses. Atomic Theory Teaching Resources | Teachers Pay Teachers There are 6 interactive activities to review Atoms and the Atomic Theory.Students will review vocabulary, scientists contributions to the atomic theory, atomic numbers and calculate mass numbers.The activities include either drag and drop or short answer text. ... Use this worksheet to help your students through an excellent 11 minute TED video ...
Atomic theory review worksheet. Atomic Theory - MRS. FURR'S WEBSITE Review Games AP Chemistry ATomic Theory: Unit 1. Atomic Theory Notes. Atomic Theory Worksheets. Atomic Theory Study Guide ... Atomic Theory Worksheets. Atomic Theory Study Guide. Pompom Isotope Activity. Flame lab questions. Powered by Create your own unique website with customizable templates. PDF Atomic Structure - Rim of the World Unified School District Atomic Mass — # of Protons — # of Neutrons — # of Electrons = 10.81 Atomic # — Atomic Mass — # of Protons # of Neutrons — # of Electrons — Atomic number equals the number of or Atomic mass equals the number of 6.941 Atomic # — Atomic Mass — # of Protons — # of Neutrons — # of Electrons — 35 Bromine 79.904 Atomic # = PDF Atomic Structure Review Worksheet Key - springtownisd.net Atomic Structure Review Worksheet Key 1. 12 found below symbol on periodic table 2. Na atomic number 11 is Sodium 3. 4 mass - protons 7-3 = 4 4. 10 protons = electrons 5. 5 found by looking for the atomic number ... True 0 atomic mass units for an electron 1 atomic mass unit for protons and neutrons 15. True The electron cloud is mainly empty ... DOC History of the Atom Worksheet - sfponline.org 1. What did J.J. Thompson discover? 2. What is the charge of an electron? 3. What are cathode rays made of? 4. Why do electrons move from the negative end of the tube to the positive end? 5. What was Thompson working with when he discovered the cathode rays? Lord Ernest Rutherford (1871 - 1937):
PDF Atomic Structure Review Worksheet - Springtown ISD Atomic Structure Review Worksheet Ions 21. What is the charge of an atom with 7 protons and 9 electrons: _____ 22. What is the charge on an ion with 3 protons and 1 electron: _____ 23. What is the charge on an ion a Chlorine ion with 17 protons and 18 electrons: _____ 24. What is the charge if there are 59 protons and 62 electrons: _____ ... Atomic Theory and Structure Review worksheet ID: 2835540 Language: English School subject: Chemistry Grade/level: 11 Age: 16-18 Main content: Atomic Theory and Structure Other contents: Unit Test Review Add to my workbooks (0) Download file pdf Embed in my website or blog Add to Google Classroom Atomic Theory - Weebly Lecture Atomic Theory. Lecture note taking outline. Conservation of Matter Lab. Atomic Structure Worksheet. Phet Simulation Build an Atom activity. Atomic Theory Worksheet Packet. Atomic Structure Practice. Atomic Theory Unit Review. Atomic History Worksheet Answers Pdf - myilibrary.org Answer Key of worksheet (History of an Atom) Answer Key of worksheet # 1 Democritus Proposes the Atom 1. The word atom comes from a Greek word that means indivisible 2. Which of the following statements is part of Democritus's theory about atoms? Atoms are the smallest piece of matter. 3. We know that Democritus was right about atoms.
PDF Basic Atomic Theory, The Structure of Matter - Appalachian State University Basic Atomic Theory, The Structure of Matter The field of study we call electricity is the investigation of the forces created by charged particles, especially electrons, and the motion and interactions of those particles. The electron is a fundamental component of matter and is considered to have the smallest possible unit of negative charge ... DOC Chapter 4 Review Worksheet - Currituck County Schools English chemist and schoolteacher who formulated a theory to describe the structure and chemical reactivity of matter in terms of atoms, 1. Who did this experiment? 2. Draw in what happened? 3. What properties did he find for the pieces? 4. How did he describe the atom? 5. Who did this experiment? 6. Describe the alpha particles, 7. PDF The Atom for Middle School - Miss Little's Classroom Website Write the letter for the atomic model in front of the statement. 1. _____ Atoms are indivisible. 2. _____ An atom is the smallest piece of matter. 3. _____ In an atom, electrons are located in energy levels that are a certain distance from the nucleus. 4. _____ Atoms are small, hard particles. 5. Atomic Structure Review Worksheet.doc - Atomic Review... Atomic Review Worksheet Section 1. Early Atomic Theory Indicate whether each statement below is TRUE or FALSE1. Ancient philosophers regularly performed controlled experiments. 2. Philosophers formulated explanations about the nature of matter based on their own experiences. 3. Both Democritus and Dalton suggested that matter is made up of atoms.
Atomic Structure Worksheet 2 Answer Key Some of the worksheets for this concept are protons neutrons and electrons practice work answer key structure of matter work answers key ebook atomic structure work 1. Atomic structure worksheet 2 answer key. In a neutral atom of that element. Use periodic table for mass. Answer key of worksheet 1 democritus proposes the atom 1.
Dalton Atomic Theory Teaching Resources | Teachers Pay Teachers This worksheet covers Dalton's Atomic Theory of Matter and the three fundamental laws of chemistry. It introduces the law of conservation of mass in a manner that prepares students for stoichiometry. ... This is a fun way to review Atomic Theory in high school. Students can work individually, pairs, or even teams. This is a great way to review ...
Mr. Christopherson / Atomic Structure - McLean County Unit District No. 5 Worksheets, * Vocabulary: Atomic Structure pdf, * Atomic Number and Mass Number pdf, * Ions and Subatomic Particles pdf, * Development of the Atomic Theory pdf (history of the Atom paragraph) * Light Problems pdf, * Half-life of Radioactive Isotopes pdf, Quantum Mechanics and Electron Configuration (paragraph) * Atom, Mass, and the Mole pdf,
PDF Atomic Theory - Review Sheet - Concord Consortium Atomic Theory - Review Sheet, You should understand the contribution of each scientist. I don't expect you to memorize dates, but I do want to know what each scientist contributed to the theory. Democretus - 400 B.C. theory that matter is made of atomsBoyle 1622 -1691, defined elementLavoisier 1743-1797,
Atomic Structure TEST REVIEW Flashcards | Quizlet 24.287. Uranium is used in nuclear reactors and is a rare element on earth. Uranium has three common isotopes. Using the chart below determine the average atomic mass of Uranium. Submit your answer with three decimal places. Isotope Mass Percent. Uranium-234 234.097 0.01. Uranium-235 235.013 0.71. Uranium-238 238.047 99.28.
Quiz & Worksheet - Atomic Theory | Study.com About This Quiz & Worksheet, By working through this assessment's multiple choice questions, you will be able to get a full evaluation of your current knowledge regarding atomic theory. The quiz...
Unit 5: Atomic Theory - Science Day 1: Review of Science 10 and Atomic Model Project

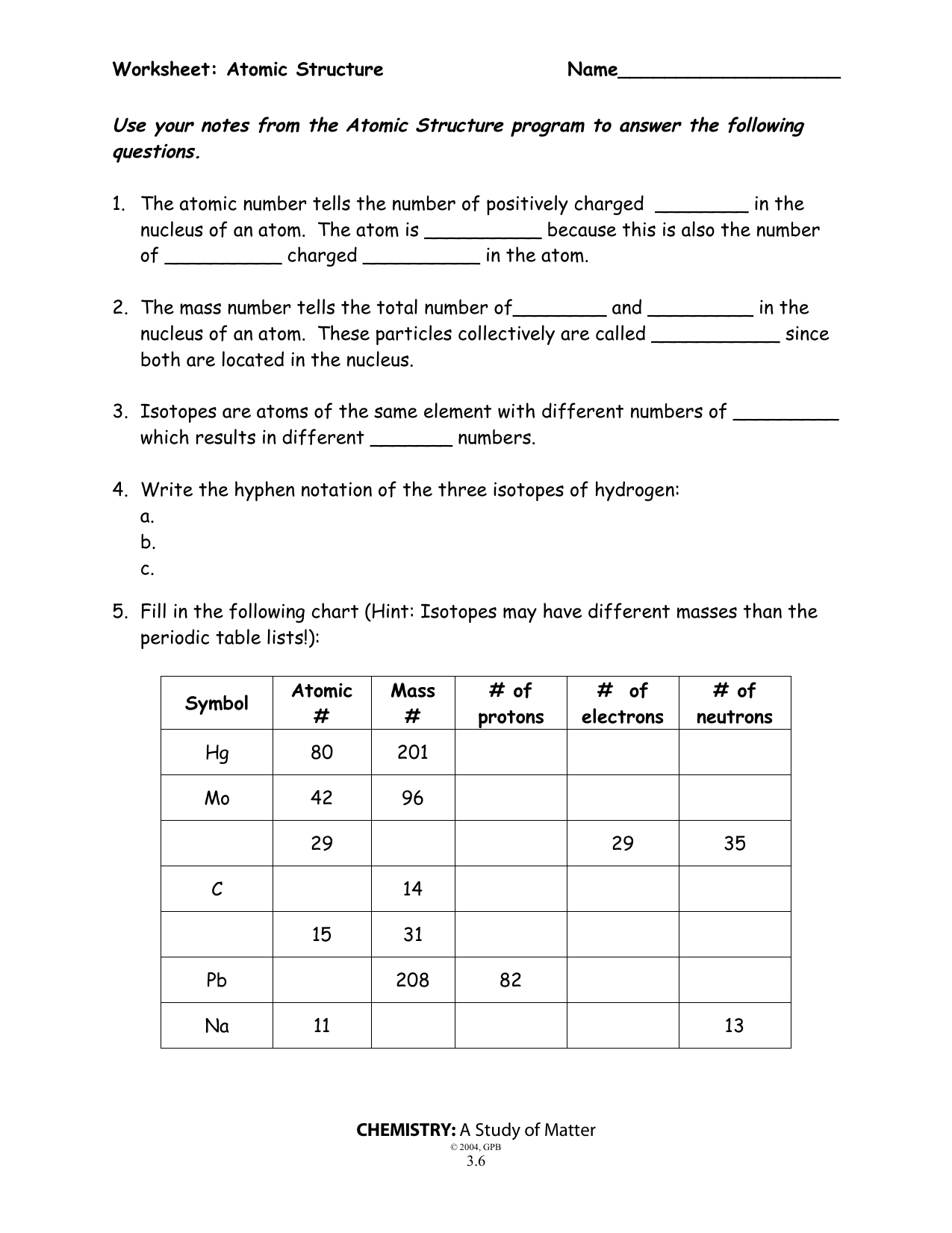



0 Response to "45 atomic theory review worksheet"
Post a Comment